Abstract
Rationale
B cell malignancies, including leukemia and lymphoma, are high-risk lymphoid neoplasms. B cell malignancies predispose to autoimmune diseases including systemic lupus erythematosus (SLE) which increase the risk of developing these malignancies by >5-fold. Increased prolactin (PRL) expression is known to exacerbate SLE and promote the survival of autoreactive B cells. Furthermore, PRL induces expression of the protooncogenes, MYC and BCL2, in lymphoid tissues. However, whether PRL drives the initiation and maintenance of B cell malignancies was not known.
Results
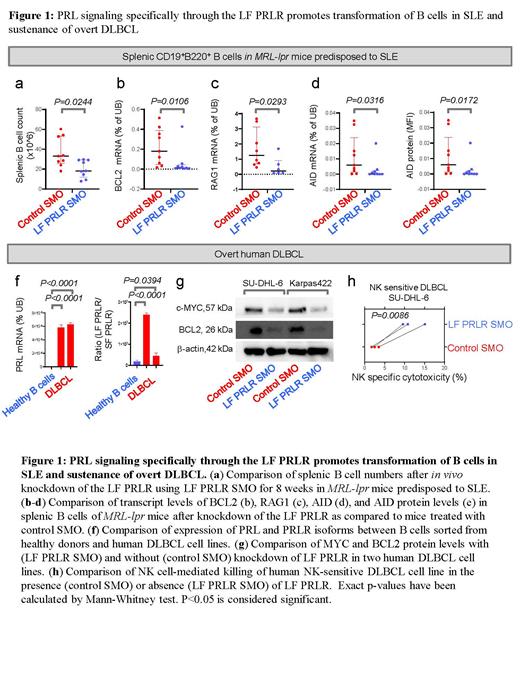
We first tested our hypothesis that PRL, specifically signaling through the pro-proliferative and anti-apoptotic long isoform (LF) of the PRL receptor (PRLR), drives the progression of SLE to B cell malignancies. To this end, we knocked down the LF PRLR in MRL-lpr mice predisposed to developing SLE using a splice-modulating oligomer (SMO) that blocks splicing to produce the LF PRLR without affecting the short isoforms. LF PRLR knockdown reduced splenic and circulating B cell numbers in MRL-lpr SLE mice (Fig.1a). Consistent with reduced B cell numbers, BCL2 expression in B cells of SLE mice was suppressed after LF PRLR knockdown, although MYC was unaltered (Fig.1b). By sequencing the immunoglobulin heavy chains (IGH), we compared the composition of the splenic B cell repertoire between control- and LF PRLR SMO-treated SLE mice. Control oligomer treated SLE mice accumulated splenic B cells with long complementary determining region 3 (CDR3) and B cells with non-functional IGH, characteristics of autoreactive B cells. Treatment with the LF PRLR SMO reduced both.
We then measured the expression of enzymes known to induce malignant transformation of B cells, namely recombination activating genes 1/2 (RAG1/2) and activation-induced cytidine deaminase (AID), in B cells of SLE mice in controls versus LF PRLR knockdown. Importantly, LF PRLR knockdown significantly reduced RAG1 (Fig.1c) and AID expression in splenic B cells of SLE mice (Fig.1d,e). Our findings thus underscore a causal role for LF PRLR signaling in promoting of malignant transformation of B cells in SLE.
Because PRL induces the expression of BCL2 and MYC in lymphocytes, we next determined whether LF PRLR promotes the survival of overt B cell malignancies that overexpress MYC and BCL2, including diffuse large B cell lymphoma (DLBCL) and B-cell acute lymphoblastic leukemia (B-ALL). We observed that B-lymphoblasts expressed significantly higher levels of PRL and the LF PRLR as compared to normal B cells (Fig.1f). We also found that higher expression of PRL at diagnosis predicts poor clinical outcome in DLBCL patients (P=0.0244), and that patients with MYC/BCL2-overexpressing ALLs with a poor prognosis had significantly higher expression of the LF PRLR compared to their MYC lowBCL2 low counterparts (P<0.0001). These observations suggested that LF PRLR may modulate MYC and BCL2 expression.
Knockdown of the LF PRLR using the LF PRLR SMO in MYC/BCL2-driven human B cell malignancies killed lymphoblasts and reduced MYC and BCL2 protein levels (Fig.1g). Because we previously showed that MYC-driven lymphoid malignancies are sensitive to natural killer (NK) cell-mediated immune clearance, we also examined whether LF PRLR knockdown synergized with NK cells in killing DLBCL. We found that LF PRLR knockdown enhanced NK cell-mediated killing of B-lymphoblasts (Fig.1h). Of note, no reductions were observed in NK cell viability or MYC levels within NK cells upon LF PRLR knockdown, suggesting that LF PRLR selectively kills B-lymphoblasts without negatively impacting NK homeostasis.
Conclusion
Our studies identify the specific knockdown of LF PRLR as a potentially safe and targeted strategy to prevent the onset of B cell malignancies in SLE patients and to treat flagrant DLBCL and B-ALL.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal